FDA Cybersecurity: Protecting Medical Devices from Cyber Threats
What do you mean by FDA Cybersecurity?
FDA Cybersecurity refers to the measures put in place by the Food and DRug Administration (FDA) to ensure the safety and security of medical devices from cyber threats. In today’s digital age, medical devices are increasingly connected to the internet, making them vulnerable to cyber attacks. These attacks can have serious consequences, ranging from the compromise of patient data to the disruption of critical medical services.
How serious is the problem?
Cybersecurity threats in the healthcare industry are on the rise, with medical devices becoming prime targets for hackers. In fact, a recent study found that 82% of healthcare organizations experienced a cyber attack in the past year. These attacks can have devastating consequences, putting patient safety at risk and compromising the integrity of medical data.
What is known about FDA Cybersecurity regulations?
The FDA has taken steps to address the growing threat of cyber attacks on medical devices. In 2018, the agency issued new guidance for medical device manufacturers on how to address cybersecurity risks. This guidance outlines the need for manufacturers to implement cybersecurity controls to protect their devices from threats.
What is the solution?
One of the key solutions to ensuring FDA Cybersecurity compliance is for manufacturers to implement a robust cybersecurity program that addresses the unique risks posed by medical devices. This program should include risk assessments, vulnerability assessments, and regular security updates to ensure that devices are protected from evolving threats.
Information on FDA Cybersecurity
Medical devices are an essential part of modern healthcare, providing critical services to patients around the world. However, the increasing connectivity of these devices has also made them vulnerable to cyber attacks. The FDA has recognized this threat and has taken steps to protect medical devices from cybersecurity risks.
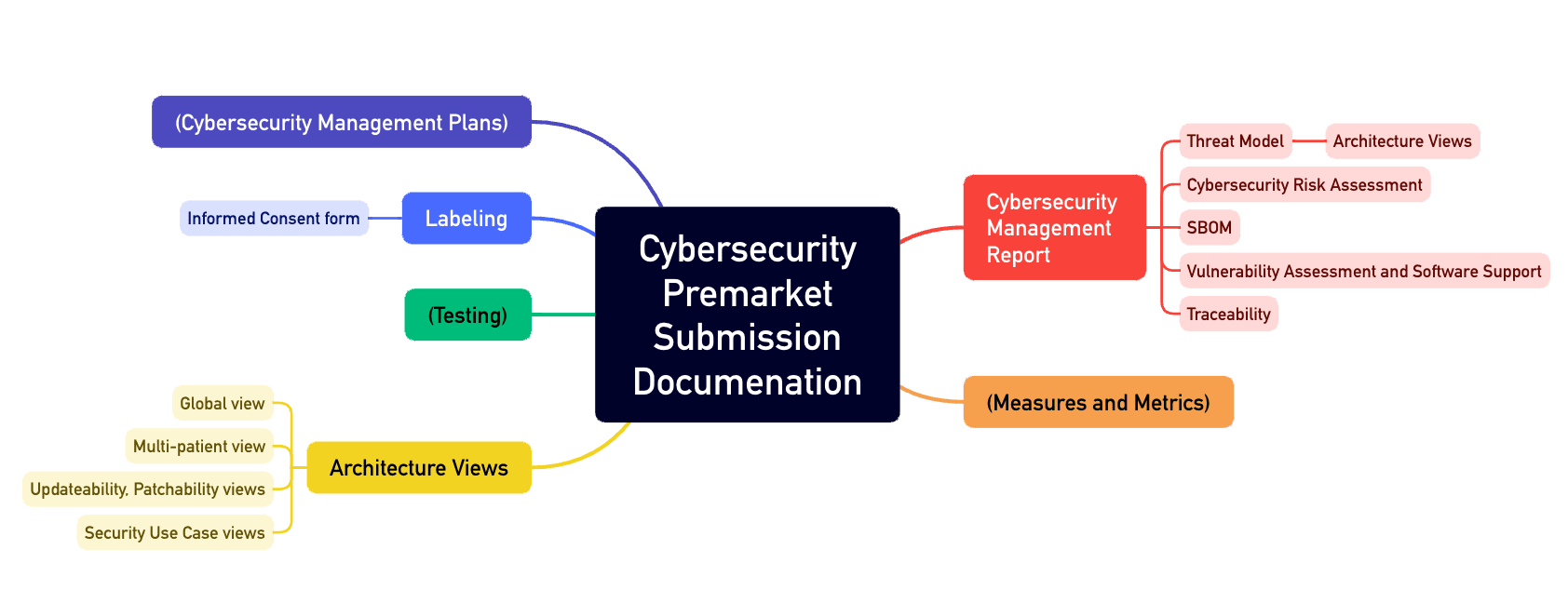
Image Source: johner-institut.de
One of the main challenges in ensuring FDA Cybersecurity compliance is the complex nature of medical devices. These devices often have unique vulnerabilities that need to be addressed through specialized cybersecurity measures. Manufacturers must work closely with cybersecurity experts to identify and mitigate these risks.
Another important aspect of FDA Cybersecurity is the need for ongoing monitoring and surveillance of medical devices. Manufacturers should have systems in place to detect and respond to cyber threats in real-time, ensuring that devices remain secure and functional at all times.
In addition to protecting devices from cyber attacks, FDA Cybersecurity regulations also aim to protect patient data. Medical devices often store sensitive information about patients, making them attractive targets for hackers. By implementing strong cybersecurity controls, manufacturers can ensure that patient data remains confidential and secure.
Overall, FDA Cybersecurity is a critical aspect of modern healthcare, ensuring that medical devices remain safe and secure in an increasingly connected world. By following the guidelines set forth by the FDA and working with cybersecurity experts, manufacturers can protect their devices from cyber threats and provide patients with the peace of mind they deserve.
Conclusion
In conclusion, FDA Cybersecurity is a vital component of modern healthcare, protecting medical devices from cyber threats and ensuring the safety and security of patients. By implementing robust cybersecurity measures and following the guidance provided by the FDA, manufacturers can safeguard their devices from evolving threats and provide patients with the care they deserve.
FAQs

Image Source: securitycompass.com
1. Why is FDA Cybersecurity important?
2. How can manufacturers ensure compliance with FDA Cybersecurity regulations?
3. What are the consequences of a cyber attack on a medical device?

Image Source: fda.gov
4. How can patients protect themselves from cybersecurity threats related to medical devices?
5. What role does the FDA play in regulating cybersecurity in the healthcare industry?
fda cybersecurity